Systems Biology
Systems Biology of Tuberculosis
Our interest in Systems Biology comes from the need of understanding many aspects of one specific case study: Tuberculosis (TB). This disease is believed to have been present in humans for thousands of years and continues to be a major cause of morbidity and mortality throughout the world. The resurgence of the disease has primarily been due to the emergence of drug resistant tubercle, especially to the most effective drug traditionally used, isoniazid and rifampicin. The number of drug-resistant cases continues to rise worldwide and it is estimated that a 5% of the cases are bacteria resistant to anti-TB drugs. Mycobacterium tuberculosis is difficult to kill for several reasons such as its slow growth, dormancy, complex cell envelope, intracellular pathogenesis and genetic homogeneity. On the other hand, a rising number of people in the developed world are contracting tuberculosis because their immune systems are compromised by immunosuppressive drugs, substance abuse, or AIDS. TB is the primary cause of death among HIV- positive. The international scientific community and the World Health Organization are aware of the need to develop new strategies and a coordinated work to tackle this problem. For this purpose, WHO has launched a global plan in order to succeed in eradicating TB by 2015. However, this is not an easy task, since it depends largely on the development of new diagnostics, drugs and vaccines for the treatment and prevention of the disease. The knowledge of the molecular basis of biological processes is of central importance to the understanding and treatment of disease and the design of new therapeutic interventions. In this sense, molecular biology has uncovered a lot of biological facts, but this is not enough for interpreting biological systems. The science of complexity has become extremely important in the study of complex biological processes, since the complex system’s perspective allows an accurate analysis of different phenomena. In our group, we use tools of Systems Biology and Statistical Physics to develop quantitative multi-scale models for the study of TB. The central objective of our research is to contribute to a better theoretical understanding of the mechanisms behind TB infection, both at the cellular and molecular levels and at the population level, where state-of-the-art epidemiological models are under development.
The study of TB & Multi-Scale Models
Simulations of biological systems have been spurred by the massive acquisition and availability of data in molecular and cell biology. In silico simulations are successfully being employed in the design of new experiments. Although the gap between in vivo and in silico biology has been reduced in the last several years, there are still many limitations hindering the adoption of computational approaches in everyday research. The Tuberculosis (TB) is a persistent infection that still causes more deaths worldwide than any other infectious disease. Roughly, one third of the human population is infected with Mycobacterium tuberculosis (M.Tb) and an estimated 5 to 10 percent of those individuals will develop TB. The need to eradicate or control TB is supported by the WHO projection on the incidence of TB by 2020: there would be 1 billion newly infected people, 200 million sick and around 35 million deaths. Tuberculosis belongs to a class of infections whose main feature is that they evolve over a large period of time by prolonged persistent interactions between the pathogen and the host. These kinds of diseases present a major challenge for disease control. Developing detailed computational models, testable predictions and guidelines for treatments aimed at lowering the incidence of persistent infections like TB may prove hard, but it is a praiseworthy effort. There are many open questions in TB research. One of the main scientific challenges is how to deal with the many spatial and time scales that are involved in M.Tb infection. To date, the majority of successful biological experiments and modeling approaches aimed at unraveling the properties and mechanisms of TB infection have been restricted to a particular level, but a full understanding calls for the integration of different scales. In this respect, the development of quantitative multi-scale models constitutes one of the major challenges.
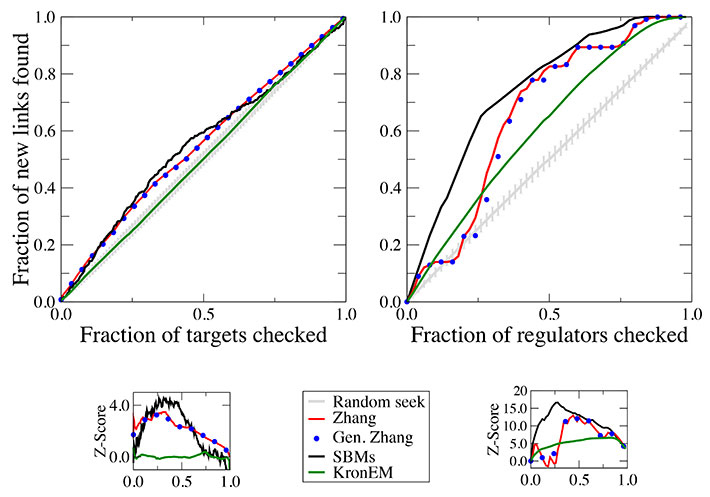 Another problem in which computational approaches could be very useful is protein function assignment. A decade ago, the TB genome was completed and published. However, we actually do not know the function of many proteins, as around 40% of the genes do not reveal any information. At the population scale, the study of how changes at lower levels are manifested at higher ones is also a challenging field of research. A key open question then is what allows for different disease outcomes following infection with M.Tb. Accurate modeling can also be applied to make quantitative predictions of the influence of different vaccination procedures and to appraise the potential impact of control programs. Moreover, the outcome of infection with M.Tb depends on many interactions that take place over various biological scales. Qualitative and quantitative modeling will be linked in order to obtain novel insights and new information to better understand the foundations of those processes involving M.Tb at several spatial and time scales. The objective will be therefore to set forth a comprehensive, integrated systems- biology toolbox specifically devised for the description of the interactions within and between the mechanisms that combine to give rise to the function and behavior of M.Tb infection. Tools derived from network science, statistical physics and dynamical systems theory, together with continuous and discrete variable models, ordinary, partial and stochastic differential equations, epidemiological approaches and agent-based modeling provide an ideal theoretical and computational foundation to reach this purpose.
Another problem in which computational approaches could be very useful is protein function assignment. A decade ago, the TB genome was completed and published. However, we actually do not know the function of many proteins, as around 40% of the genes do not reveal any information. At the population scale, the study of how changes at lower levels are manifested at higher ones is also a challenging field of research. A key open question then is what allows for different disease outcomes following infection with M.Tb. Accurate modeling can also be applied to make quantitative predictions of the influence of different vaccination procedures and to appraise the potential impact of control programs. Moreover, the outcome of infection with M.Tb depends on many interactions that take place over various biological scales. Qualitative and quantitative modeling will be linked in order to obtain novel insights and new information to better understand the foundations of those processes involving M.Tb at several spatial and time scales. The objective will be therefore to set forth a comprehensive, integrated systems- biology toolbox specifically devised for the description of the interactions within and between the mechanisms that combine to give rise to the function and behavior of M.Tb infection. Tools derived from network science, statistical physics and dynamical systems theory, together with continuous and discrete variable models, ordinary, partial and stochastic differential equations, epidemiological approaches and agent-based modeling provide an ideal theoretical and computational foundation to reach this purpose.
Find out more by checking our main publications at: MAIN PUBLICATIONS

